A4 - Innervation of adipose tissue: identifying players and their function
This project has been funded by the DFG from 2013 to 2016. It is continued with a new scope in CRC project B9.
Obesity, the excessive accumulation of white adipose tissue (WAT) is highly prevalent in western societies and associated with increased risk for type II diabetes, hyperlipidemia, hypertension, and even certain forms of cancer. Despite of its clinical importance, our knowledge of control over WAT development, maintenance and degradation is extremely limited. While it is known that altered innervation of WAT strongly impacts on its architecture, the underlying mechanisms are also ill-defined. Combining morphological analysis and (calcium) imaging of live, intact preparations, this project is designed to i) identify functionally active neurotransmitters and localize their receptors in WAT including its vessels, ii) establish their effects in regard to WAT function, architecture and recruitment of adipocytes precursors from the perivascular compartment, and iii) test whether these precursors are replenished from bone marrow cells. The leading hypothesis is that innervation impacts on both, metabolism and fat storage as well as on development of new adipocytes.
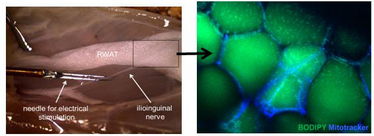
Figure 1. Simultaneous electrical stimulation of peripheral nerves running into the retroperitoneal fat pad (RWAT) and quantitative microscopy will gain insight into the direct target cells of adipose tissue innervation and their function. Adipocytes are visible after lipid (Bodipy) and mitochondria (Mitotracker) staining.
Bechor S, Nachmias D, Elia N, Haim Y, Vatarescu M, Leikin-Frenkel A, Gericke M, Tarnovsky T, Las G, Rudich A. Adipose tissue conditioned media support macrophage lipid-droplet biogenesis by interfering with autophagic flux. Biochim Biophys Acta. 2017; Epub ahead of print.
Braune J, Weyer U, Matz-Soja M, Hobusch C, Kern M, Kunath A, Klöting N, Kralisch S, Blüher M, Gebhardt R, Zavros Y, Bechmann I, Gericke M. Hedgehog sinaling in myeloid cells impacts on body weight, adipose tissue inflammation and glucose metabolism. Diabetologia. 2017;60:889-99.
Stelzner K, Herbert D, Popkova Y, Lorz A,Schiller J, Gericke M, Klöting N, Blüher M, Franz S, Simon JC, Saalbach A. Free fatty acids sensitize dendritic cells to amplify TH1/TH17-immune responses. Eur J Immunol. 2016;46:2043-53.
Orthgiess J, Gericke M, Immig K, Schulz A, Hirrlinger J, Bechmann I, Eilers J. Neurons exhibit Lyz2 promoter activity in vivo: Implications for using LysM-Cre mice in myeloid cell research. Eur J Immunol. 2016;46:1529-32.
du Plessis J, van Pelt J, Korf H, Mathieu C, van der Schueren B, Lannoo M, Oyen T, Topal B, Fetter G, Nayler S, van der Merwe T, Windmolders P, van Gaal L, Verrijken A, Hubens G, Gericke M, Cassiman D, Francque S, Nevens F, van der Merwe S. Association of adipose tissue inflammation with histological severity of non-alchoholic fatty liver disease. Gastroenterology. 2015;149:635-48.
Gericke M, Weyer U, Braune J, Bechmann I, Eilers J. A method for long-term live-imaging of tissue macrophages in adipose tissue explants. Am J Physiol Endocrinol Metab. 2015;308:E1023-33.
Kälin S, Heppner FL, Bechmann I, Prinz M, Tschöp MH, Yi CX. Hypothalamic innate immune reaction in obesity. Nat Rev Endocrinol. 2015;11:339-51.
Koch M, Varela L, Kim JG, Kim JD, Hernandez-Nuno F, Simonds SE, Castorena CM, Vianna CR, Elmquist JK, Morozov YM, Rakic P, Bechmann I, Cowley MA, Szigeti-Buck K, Dietrich MO, Gao XB, Diano S, Horvath TL. Hypothalamic POMC neurons promote cannabinoid-induced feeding. Nature. 2015;519:45-50.
Stenkamp-Strahm CM, Nyavor YE, Kappmeyer AJ, Horton S, Gericke M, Balemba OB. Prolonged high fat diet ingestion, obesity, and type 2 diabetes symptoms correlate with phenotypic plasticity in myenteric neurons and nerve damage in the mouse duodenum. Cell Tissue Res. 2015;361:411-26.
Berry R, Church CD, Gericke MT, Jeffery E, Colman L, Rodeheffer MS. Imaging of adipose tissue. Methods Enzymol. 2014;537:47-73.
Haase J, Weyer U, Immig K, Klöting N, Blüher M, Eilers J, Bechmann I, Gericke M. Local proliferation of macrophages in adipose tissue during obesity-induced inflammation. Diabetologia. 2014;57:562-71.
Kosacka J, Nowicki M, Blüher M, Baum P, Stockinger M, Toyka KV, Klöting I, Stumvoll M, Serke H, Bechmann I, Klöting N. Increased autophagy in peripheral nerves may protect Wistar Ottawa Karlsburg W rats against neuropathy. Exp Neurol. 2013;250:125-35.
Gericke MT, Schröder T, Kosacka J, Nowicki M, Klöting N, Spanel-Borowski K. Neuropeptide Y impairs insulin-stimulated translocation of glucose transporter 4 in 3T3-L1 adipocytes through Y1 receptor. Mol Cell Endocrinol. 2012;348:27-32.
Prodinger C, Bunse J, Krüger M, Schiefenhövel F, Brandt C, Laman JD, Greter M, Immig K, Heppner F, Becher B, Bechmann I. CD11c-expressing cells reside in the juxtavascular parenchyma and extend processes into the glia limitans of the mouse nervous system. Acta Neuropathol. 2011;121:445-58.
Hallermann S, Fejytova A, Schmidt H, Weyhersmüller A, Silver RA, Gundelfinger ED, Eilers J. Bassoon speeds vesicle reloading at a central excitatory synapse. Neuron. 2010;68:710-23.
Gericke MT, Kosacka J, Koch D, Nowicki M, Schröder T, Ricken AM, Nieber K, Spanel-Borowski K. Receptors for NPY and PACAP differ in expression and activity during adipogenesis in the murine 3T3-L1 fibroblast cell line. Br J Pharmacol. 2009;157:620-32.
Gao Q, Mezei G, Nie Y, Rao Y, Choi CS, Bechmann I, Leranth C, Toran-Allerand D, Priest CA, Roberts JL, Gao XB, Mobbs C, Shulman GI, Diano S, Horvath TL. Anorectic estrogen mimics leptin's effect on the rewiring of melanocortin cells and Stat3 signaling in obese animals. Nat Med. 2007;13:89-94.
Wilms CD, Schmidt H, Eilers J. Quantitative two-photon Ca2+ imaging via fluorescence lifetime analysis. Cell Calcium. 2006;50:73-9.
Bechmann I, Goldmann J, Kovac AD, Kwidzinski E, Simbürger E, Naftolin F, Dirnagl U, Nitsch R, Priller J. Circulating monocytic cells infiltrate layers of anterograde axonal degeneration whre they transform into microglia. FASEB J. 2005;19:647-9.
Priller J, Flügel A, Wehner T, Boentert M, Haas CA, Prinz M, Fernandez-Klett F, Prass K, Bechmann I, de Boer BA, Frotscher M, Kreutzberg GW, Persons DA, Dirnagl U. Targeting gene-modified hematopoietic cells to the central nervous system: use of green fluorescent protein uncovers microglial engraftment. Nat Med. 2011;7:1356-61.
Garaschuk O, Linn J, Eilers J, Konnerth A. Large-scale oscillatory calcium waves in the immature cortex. Nat Neurosci. 2000;3:452-9.
Takechi H, Eilers J, Konnerth A. A new class of synaptic response involving calcium release in dendritic spines. Nature. 1998;396:757-60.
PROJECT TEAM
| Prof. Dr. Ingo Bechmann Leipzig University Faculty of Medicine Institute of Anatomy Liebigstrasse 13 04103 Leipzig e-mail: ingo.bechmann@medizin.uni-leipzig.de Phone: 0341 / 97-22000 |
Prof. Dr. Jens Eilers |